Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
The first FDA-cleared device that supports concurrent reading, allowing for faster reading with proven, superior, automatic nodule detection.
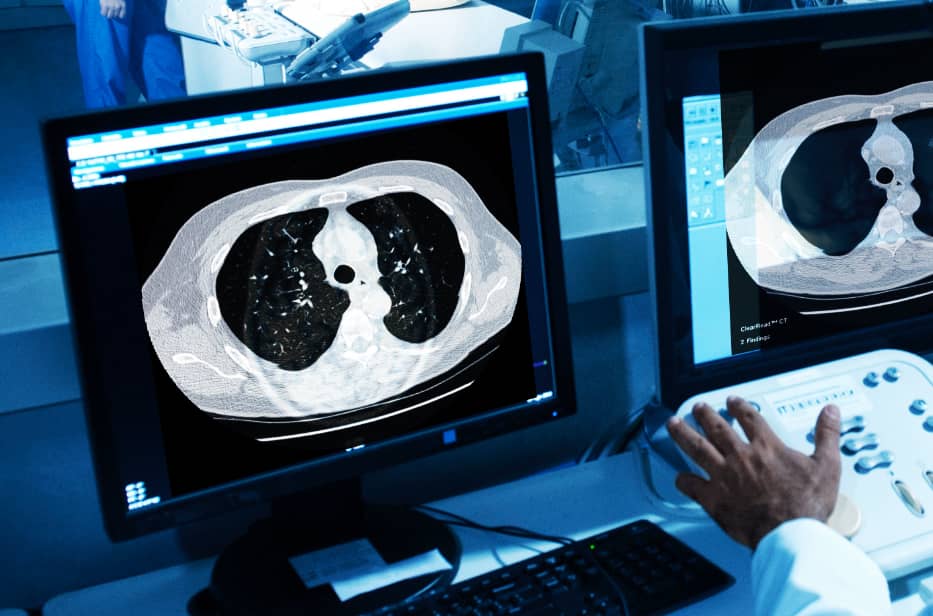
Our five FDA-cleared applications are designed to improve reading efficiency and accuracy across the hospital enterprise without additional equipment, procedures or radiation dose.
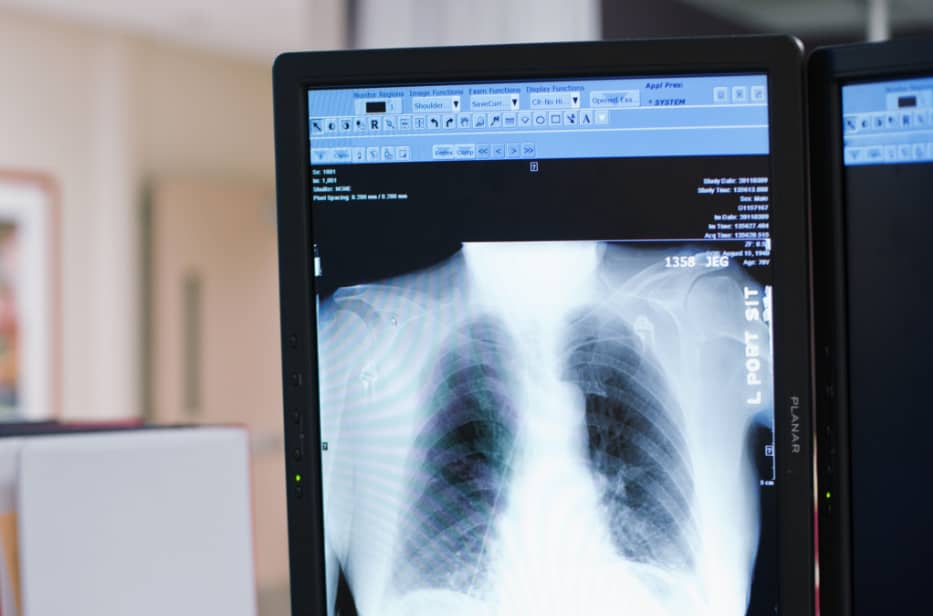
Riverain’s deep learning AI technology is FDA cleared, field tested, and clinically proven to provide real results in radiology.
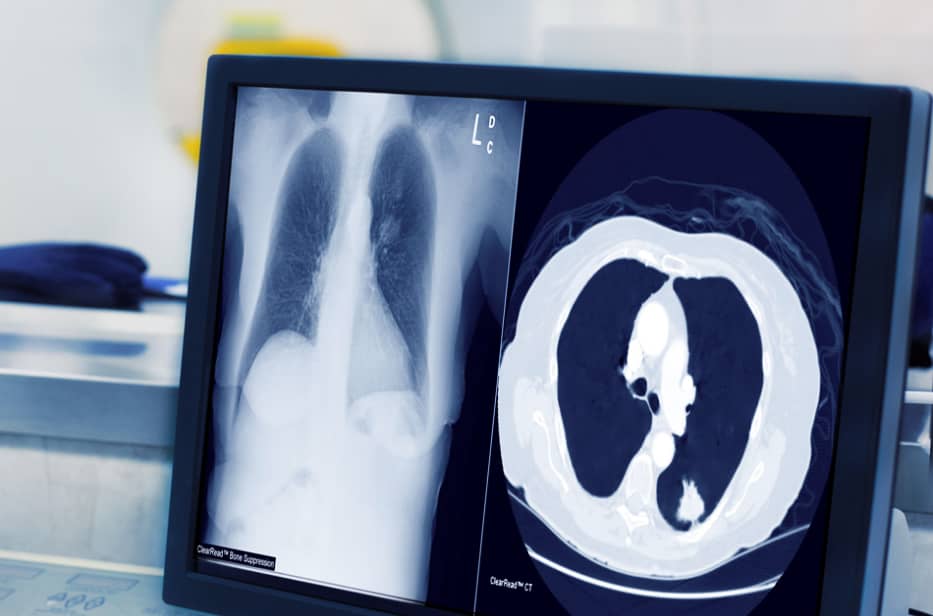
Riverain Technologies advanced AI imaging software is in use by leading healthcare organizations around the world.

Peer reviews, reference accounts, and independent studies confirm that ClearRead technology is positively impacting the field of radiology.
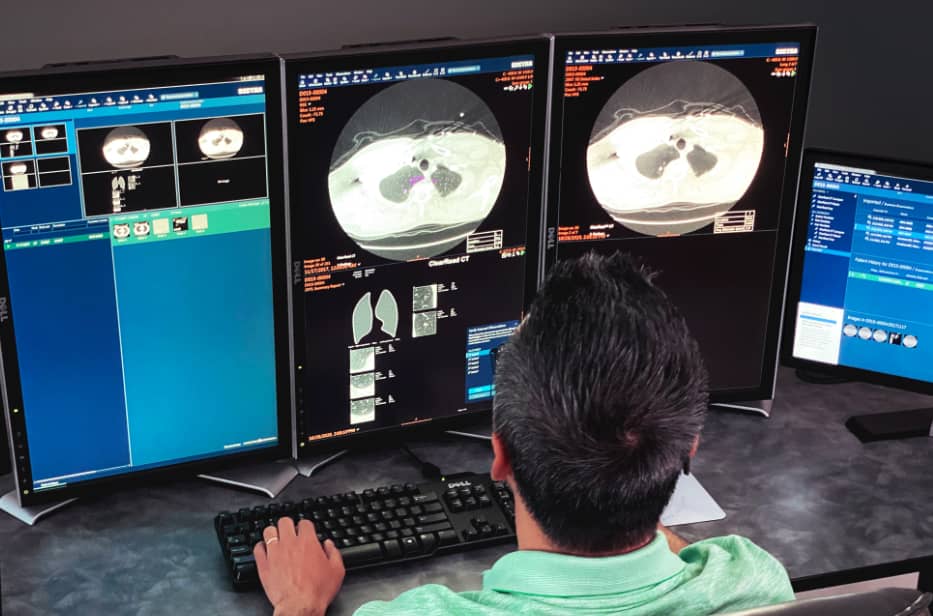
Managing your lung program can seem daunting, whether you’re getting going, growing, or managing workflow. We’ve curated resources for you.
DAYTON, Ohio – September 26, 2013 – Riverain Technologies is pleased to announce that it has received approval from the China Food and Drug Administration (CFDA) for their ClearRead Bone Suppression (formerly SoftView) and Computer Aided Detection Software, ClearRead +Detect, (formerly OnGuard). With this approval, Riverain Technologies is now able to provide advanced lung disease detection applications to the Chinese market.
China has many factors that contribute to lung disease, such as a large population of heavy smokers, air pollution, and a high volume of the population exposed to toxins, pollutants, smog and chemicals in the air from poor working and living conditions. 1 The lung cancer incidence rate in Beijing has risen by 56 percent from 2001 to 2010, with most of those resulting in death.2
With the use of Riverain’s Bone Suppression and Computer Aided Detection software, lung disease can be identified and treated sooner. Riverain’s Bone Suppression image aids in the detection of 1 in 6 nodules that were previously missed3 and Riverain’s Computer Aided Detection software has been proven to identify as many as 1 in 2 nodules that were previously missed.4
“With the increasing incidence of lung cancer and complex addressability issues of the Chinese market, having cost effective, broadly accessible tools that make the most of existing technology is critical,” said Riverain CEO, Steve Worrell. “Riverain Technologies’ software provides radiologists with additional diagnostic information that allows them to detect and treat their patients earlier, when survival rates are highest.”
Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
Subscribe to Riverain via Email
Stay up to date on the latest advancements
Riverain Technologies
3130 South Tech Blvd., Miamisburg, OH 45342
800-990-3387
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |