Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
The first FDA-cleared device that supports concurrent reading, allowing for faster reading with proven, superior, automatic nodule detection.
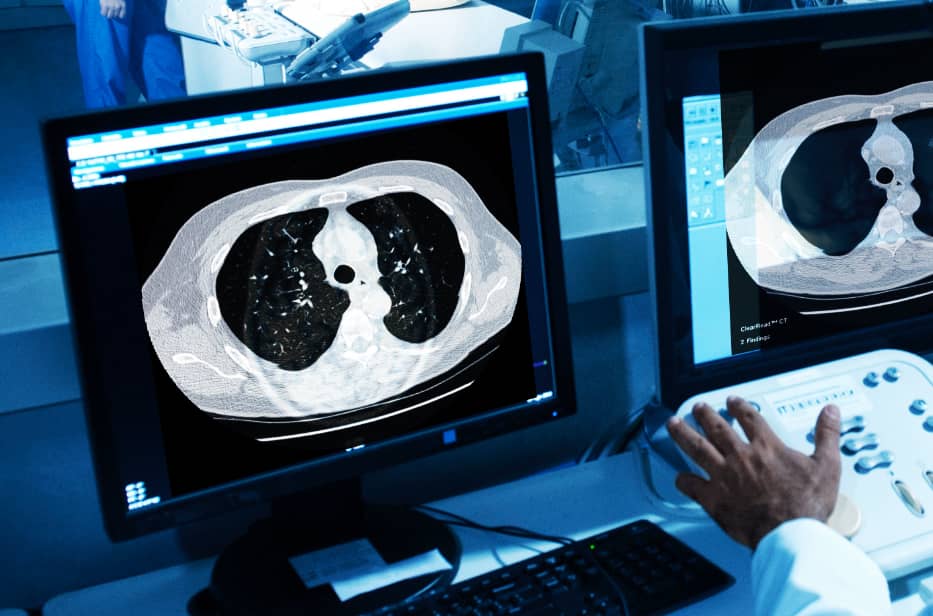
Our five FDA-cleared applications are designed to improve reading efficiency and accuracy across the hospital enterprise without additional equipment, procedures or radiation dose.
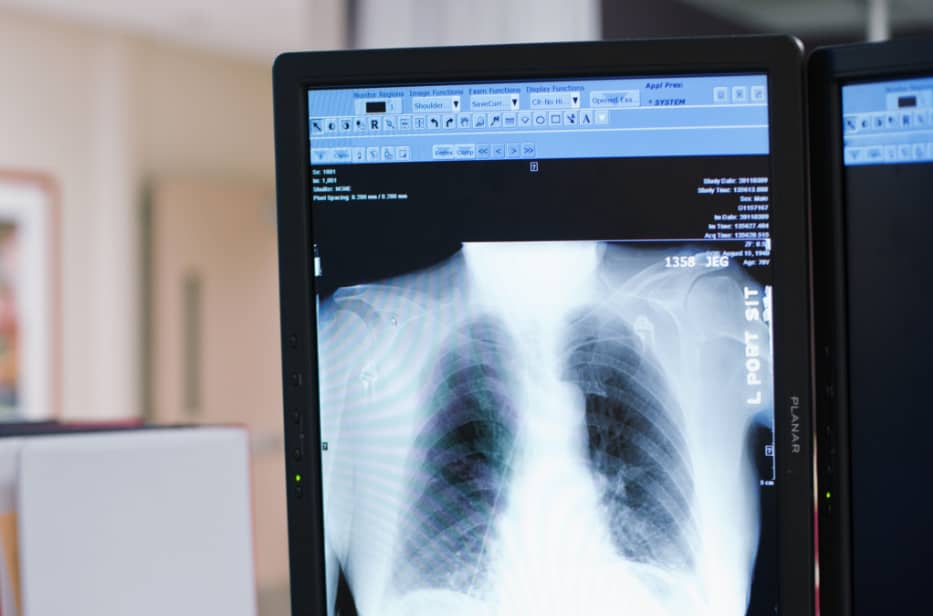
Riverain’s deep learning AI technology is FDA cleared, field tested, and clinically proven to provide real results in radiology.
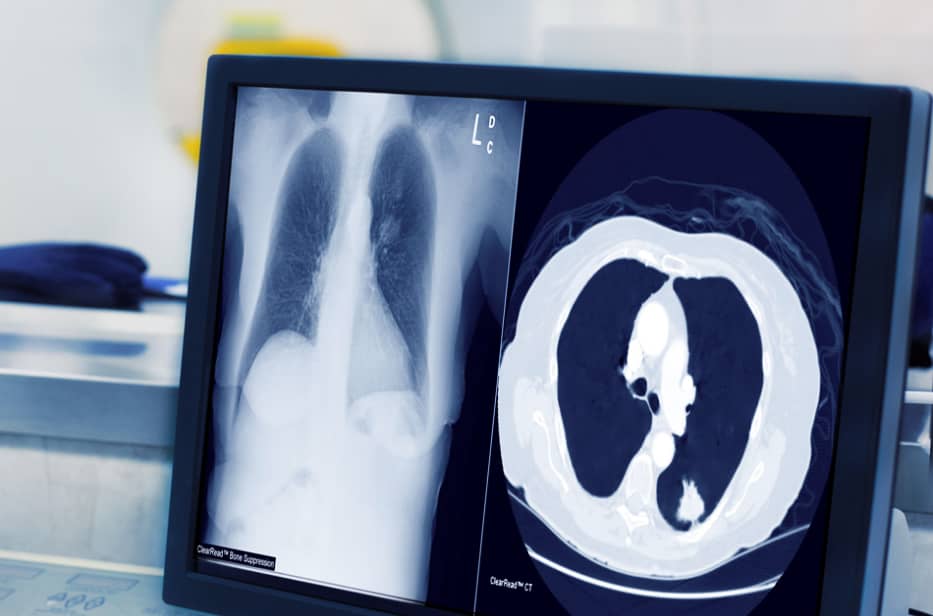
Riverain Technologies advanced AI imaging software is in use by leading healthcare organizations around the world.

Peer reviews, reference accounts, and independent studies confirm that ClearRead technology is positively impacting the field of radiology.
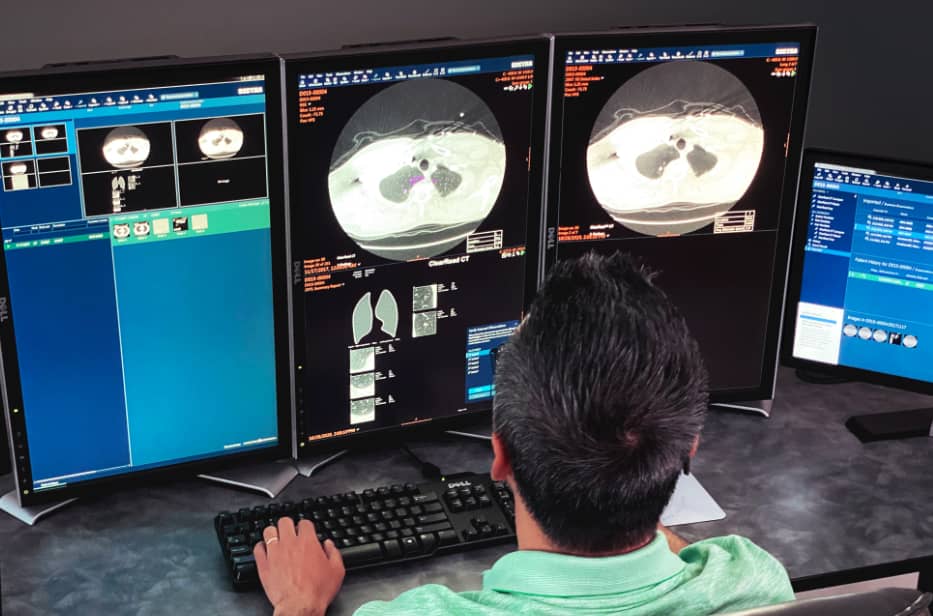
Managing your lung program can seem daunting, whether you’re getting going, growing, or managing workflow. We’ve curated resources for you.

MIAMISBURG, Ohio (March 18, 2025) – Riverain Technologies is pleased to announce that Health Canada has approved ClearRead CT CAC, an advanced artificial intelligence solution for opportunistic coronary artery calcification (CAC) quantification on ungated, non-contrast chest CT exams. This approval paves the way for Canadian radiologists to leverage ClearRead CT CAC to identify and quantify coronary artery calcifications efficiently and accurately, aiding in early detection and risk stratification of cardiovascular disease.
ClearRead CT CAC is a part of the ClearRead CT suite of AI-powered thoracic imaging solutions. ClearRead CTsuppresses vessels for an unimpaired view of the chest. Then, it detects, segments, characterizes and measures nodules. ClearRead CT CAC builds on this foundation by enabling an automated, accurate Agatston score and risk classification in routine chest CT scans, providing valuable insights into a patient’s cardiovascular health without the need for dedicated cardiac imaging.
Historically, coronary artery calcium exams have only been offered to symptomatic patients using specialized CT scans. This has overlooked the opportunity for early diagnosis. ClearRead CT CAC accurately detects, quantifies, and reports an Agatston score. It is compatible with ungated, non-contrast standard and low-dose chest CT exams, making it a valuable addition to lung cancer screening programs. Patients who undergo a CT scan for unrelated reasons can also benefit from ClearRead CT CAC scoring.
“Coronary artery disease affects over 300 million people worldwide each year*,” said Steve Worrell, CEO, Riverain Technologies. “We are thrilled to receive Health Canada approval for ClearRead CT CAC, allowing us to expand access to this powerful tool for Canadian radiologists and healthcare providers to enable earlier disease detection.”
Riverain Technologies will be showcasing ClearRead CT at the Canadian Association of Radiologists (CAR) Annual Scientific Meeting, April 3-6, 2025, in Montreal. The company will be exhibiting in the Microsoft booth, demonstrating how its AI-driven imaging solutions integrate seamlessly into radiology workflows, helping clinicians make faster, more accurate decisions.
About Riverain Technologies
Riverain Technologies is dedicated to transforming the field of radiology by addressing and eliminating delayed cardiothoracic diagnoses. As relentless innovators, Riverain empowers healthcare providers by streamlining diagnostic workflows, enhancing detection accuracy, and ultimately improving patient outcomes. With a steadfast commitment to advancing cardiothoracic care, Riverain Technologies is shaping the future of diagnostic excellence. For more information: https://www.riveraintech.com/
Sources:
*https://www.jacc.org/doi/10.1016/S0735-1097%2824%2904310-9#:~:text=In%202022%2C%20there%20were%20315,to%205445)%20per%20100000).
Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
Subscribe to Riverain via Email
Stay up to date on the latest advancements
Riverain Technologies
3130 South Tech Blvd., Miamisburg, OH 45342
800-990-3387
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
Notifications