Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
The first FDA-cleared device that supports concurrent reading, allowing for faster reading with proven, superior, automatic nodule detection.
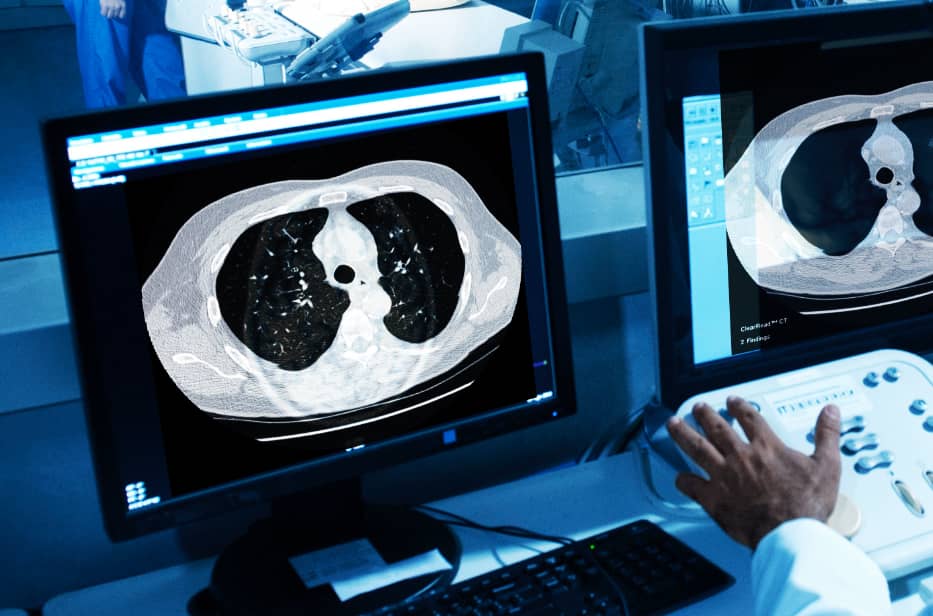
Our five FDA-cleared applications are designed to improve reading efficiency and accuracy across the hospital enterprise without additional equipment, procedures or radiation dose.
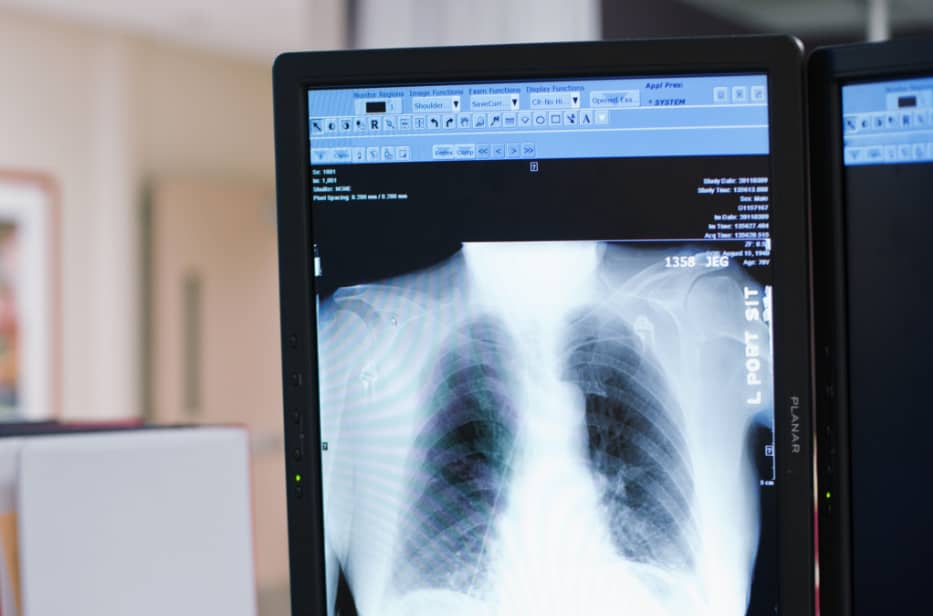
Riverain’s deep learning AI technology is FDA cleared, field tested, and clinically proven to provide real results in radiology.
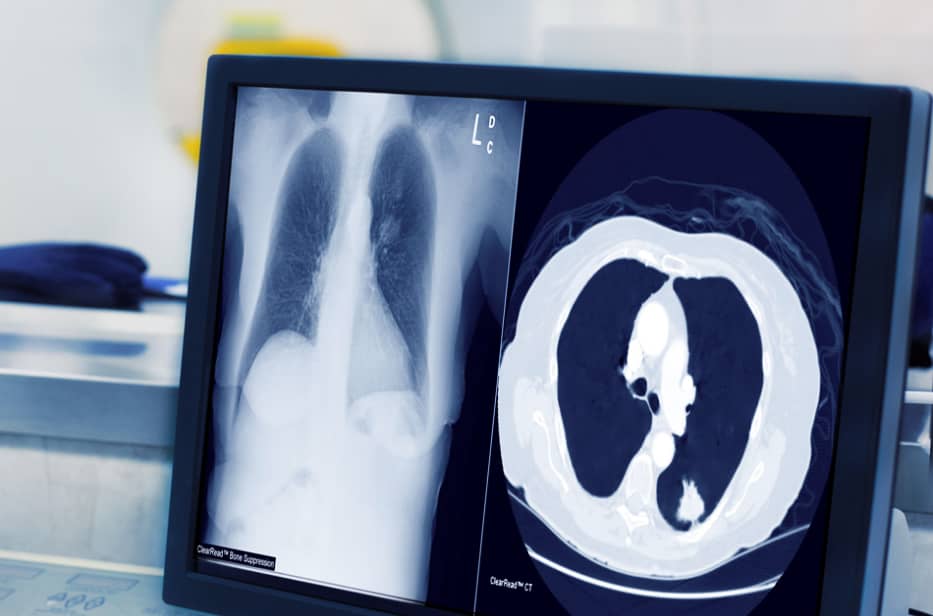
Riverain Technologies advanced AI imaging software is in use by leading healthcare organizations around the world.

Peer reviews, reference accounts, and independent studies confirm that ClearRead technology is positively impacting the field of radiology.
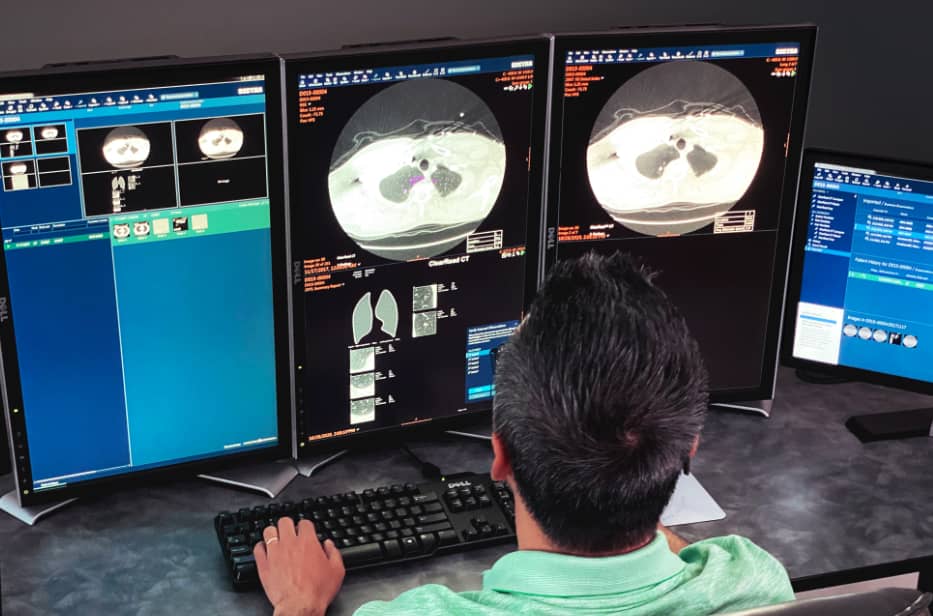
Managing your lung program can seem daunting, whether you’re getting going, growing, or managing workflow. We’ve curated resources for you.
DAYTON, Ohio – June 13, 2018 – Riverain Technologies, an industry leader and innovator in proprietary image analysis and machine learning technologies, announced today that the United States Patent and Trademark Office (USPTO) has awarded the company a broad patent for its technology that forms a vessel suppressed, chest CT series using deep learning and synthetic data modeling. The patent holds enormous promise for improving the detection and diagnosis of lung cancer at an earlier stage while making clinicians more efficient, a first for computer aided detection.
Riverain has been issued U.S. Patent 9,990,743 for its methodology to selectively remove anatomical structures from CT studies to optimize reading accuracy and efficiency for lung cancer detection. The technology is used in the company’s premier software, ClearRead CT. ClearRead CT is proven to lead to more accurate and efficient detection of lung nodules, the early indicator of lung cancer. A range of possible applications exist within other imaging modalities including MRI, positron emission tomography (PET), full field digital mammography and tomosynthesis, where normal structures interfere with the detection and diagnosis of disease.
Riverain Technologies is a leader in the commercialization of Clinical AI, offering five clinically proven FDA cleared products that incorporate state-of-the-art machine learning technologies.
Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
Subscribe to Riverain via Email
Stay up to date on the latest advancements
Riverain Technologies
3130 South Tech Blvd., Miamisburg, OH 45342
800-990-3387
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |